This week is the seventh annual #MedSafetyWeek, a global campaign aimed at encouraging people to report medication that makes them ill or causes serious side effects etc. If we don’t do this, we can’t hope to help others. If people don’t use the reporting procedure, medication could continue to affect others.
In this blog you’ll find out how to report an adverse drug reaction. It’s never too late to do so either, if you think something happened a while ago, still report it. But the sooner the better so we can affect real, meaningful and timely changes towards better safety.

Report suspected adverse reactions to medicines
This year’s campaign theme is “how patients and healthcare professionals make safety work” and the focus is on the importance of reporting suspected adverse reactions to medicines and vaccines as soon as they arise. We are also encouraging the reporting of suspected problems with medical devices or other healthcare products to the Yellow Card scheme and local reporting systems.
Help improve the safety of medicines and medical services
Running from the 7th until 13 November, this campaign aims encourage widespread public engagement and improve patient safety by reporting suspected side effects associated with medicines and adverse incidents involving medical devices.
“Every report to MHRA’s Yellow Card scheme helps improve the safety of medicines and medical devices for all.”
Medicines and Healthcare products Regulatory Agency (MHRA)
Together we can improve drug safety
This year’s campaign is a truly global effort and involves healthcare product regulators from no fewer than 82 countries. It focuses on the vital role played by every healthcare professional, patient, and carer who reports a suspected side effect or adverse incident, which in turn supports the safe use of medicines and medical devices.
They’re not telepathic – please report adverse reactions
All medicines may cause side effects and adverse incidents may occur during the use of medical devices, so it is important to have robust measures in place to continuously monitor their safety after they are taken into clinical use. The purpose of safety monitoring is to gain more information about known side effects and adverse incidents, to find out about new ones, and, most importantly, to make use of medicines and medical devices as safe as possible. Regulators operate systems to detect and analyse those side effects and adverse incidents and prevent harm to future patients.
It’s not too late – report adverse reactions today
It is important that everyone makes a report as soon as they suspect side effects and adverse incidents. This ensures that regulatory assessments are genuinely representative and can improve safety for as many people as possible.
Introducing the Yellow Card Scheme (in the UK)
The Yellow Card scheme is the MHRA’s single system for collecting suspected side effects of medicines and adverse incidents involving medical devices. These side effects and adverse incidents are then collated and swiftly investigated by the MHRA. Since its establishment in 1964 the scheme has identified numerous safety issues to the benefit of many, thanks to individual reports from medicines users across the nation.
Minimum information required for the Yellow Card Form
You’ll find an online form to fill in when you visit the Yellow Card scheme and it can be quite daunting. You will need the information below before you proceed:
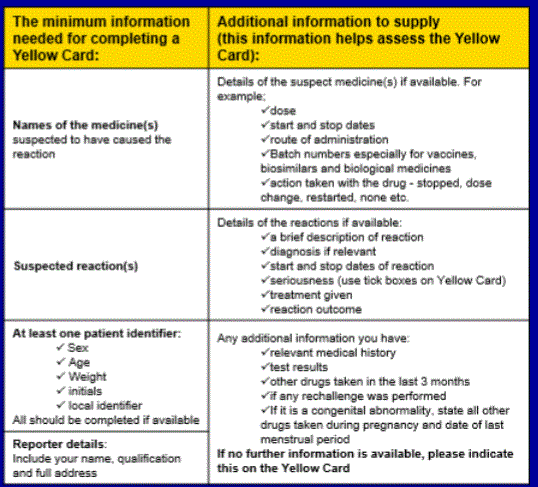
You can also request your medical history from your local GP practise if you can’t remember dates etc. which will help you complete the form accurately.
You can help protect against further harm
Everyone who reports suspected side effects and adverse incidents to the Yellow Card scheme website or app, is actively participating in identifying emerging national safety issues, so that the MHRA can act when necessary and protect against harm.
Phil Tregunno, MHRA Deputy Director of Patient Safety Monitoring, said:
Every report made by a patient, a healthcare professional, or a carer plays a key role in gaining knowledge about the risks of medicines and medical devices in clinical use and allows rapid, targeted action to be taken to minimise harm and ensure that the benefit-risk remains favourable.
Reporting suspected side effects and adverse incidents to the Yellow Card website is not just about the individual involved, it helps to improve the safety of medicines and medical devices for all patients. By reporting, you are part of the solution – and you may be helping to protect the most vulnerable, at-risk groups from potential harm.
If you, your child, or a patient in your care experiences a suspected side effect or adverse incident relating to a medicine or medical device, it is essential that you report it to us promptly. The faster you report, the likelier it is that we can intervene and prevent others from experiencing untoward, serious, life-altering, and occasionally life-threatening issues.
Minister of State for Health and Secondary Care, Will Quince, said:
Patient safety is at the heart of ensuring the best care, and this government takes it extremely seriously. I’m pleased to support this campaign which will help to further improve the safety of medicines in the UK.
I urge everyone from healthcare professionals to patients to continue to report any adverse effects involving medicines or medical devices through the Yellow Card scheme, so potential incidents can be investigated quickly.
Here’s how to use the Yellow Card Scheme
Anyone can report suspected side effects and adverse incidents to the Yellow Card scheme. Reports can be submitted in several ways:
- Online at www.mhra.gov.uk/yellowcard
- Via the free Yellow Card app (available for Android and Apple devices)
- By phone on 0808 100 3352
- By paper forms available at pharmacies and GP surgeries
Healthcare professionals and providers can also use local clinical systems to make a report, such as MiDatabank, SystmOne, or VISION.
Reports related specifically to COVID-19, including suspected side effects caused by medicines, vaccines, and medical device and incidents when using coronavirus test kits should be made at coronavirus-yellowcard.mhra.gov.uk
Notes to Editors
- National medicines regulators from 82 countries across the globe and their stakeholders will be taking part in this international campaign led by Uppsala Monitoring Centre (UMC) – the World Health Organisation (WHO) Collaborating Centre for International Drug Monitoring. The campaign is supported by members of the International Coalition of Medicines Regulatory Authorities (ICMRA). The #MedSafetyWeek 2022 project team consists of representatives from the following organisations working collaboratively: the Medicines and Healthcare products Regulatory Agency (UK) as co-lead, the Health Products Regulatory Authority (Ireland), the International Society of Pharmacovigilance (ISoP) Egypt Chapter, and the Colombian Pharmacovigilance Association.
- The Medicines and Healthcare products Regulatory Agency (MHRA) regulates medicines, medical devices and blood components for transfusion in the UK. MHRA is an executive agency, sponsored by the Department of Health and Social Care.
- Patients using the Yellow Card site should type the relevant medicine, vaccine, or medical device into the search bar and select ‘Start report’ on the right-hand side of the bar. The search bar also offers a drop-down menu to report for medicines, vaccines, medical devices, blood factors and immunoglobulin products, E-cigarettes, and herbal or homeopathic medicines that are not on the MHRA’s list, as well as the option to send additional details of the suspected material(s) if the product type is unknown, so that the final report is accurate.
- For adverse incidents associated with a medical device, healthcare professionals should report through the Yellow Card scheme or their local clinical reporting systems. Healthcare professionals in Northern Ireland and Scotland will need to report adverse incidents involving medical devices through the Northern Ireland Adverse Incident Centre (NIAIC) or National Services Scotland.
- Patients are advised to contact a healthcare professional if they are worried about their health or the safety of any healthcare product they are receiving.
Have you reported an adverse reaction?
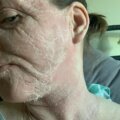
I have previously reported my own experiences using topical steroids for eczema since long term use of topical corticosteroids caused my skin to become addicted, leaving me in a rebound flare cycle that I couldn’t get out of. Having almost reached four years without using any topical steroids and with my skin continuing to heal, sharing this experience with others is important to me.
It’s also a good way to feel you are making a difference and helping others. Have you ever reported a medication? I’d love to hear your stories. What did you report? How did it make you feel? Have you seen change from drug reporting using the Yellow Card Scheme?











Leave a Reply